This is how South Africa’s vaccination programme works. But is the system able to scale up?
Getting vaccinated “is the best thing you can do for yourself” says Groote Schuur nurse
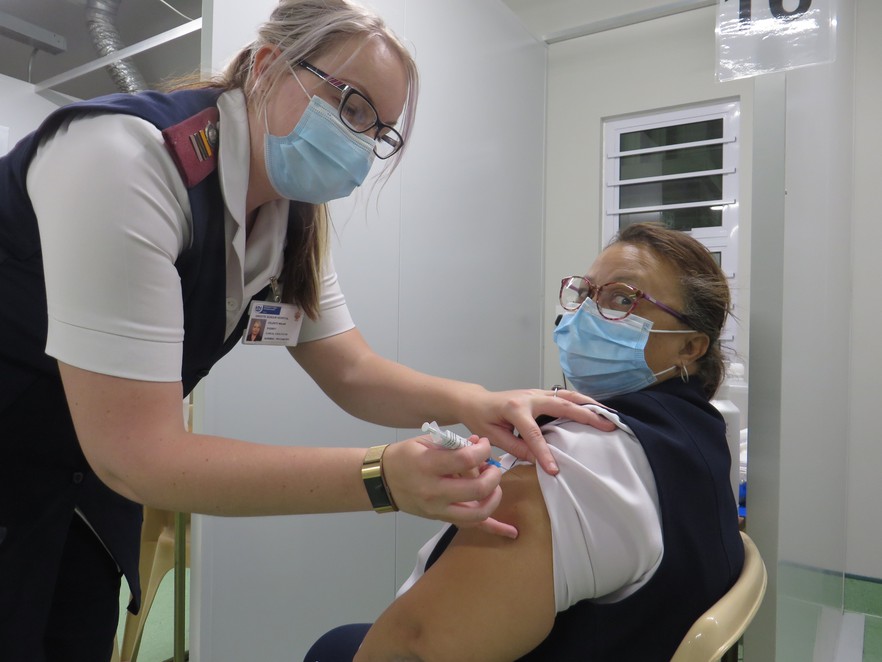
Sister Celente Malan, one of the trained vaccinators at Groote Schuur Hospital, shows how a vaccination is done. This is a mock-up: Sister Rowena Jacobs, who was vaccinated three weeks ago, is also one of the vaccinators. The hospital has 18 vaccination booths. Photo: Elsabé Brits
- Vaccine storage, cold chain distribution and vaccinating is stringently controlled.
- Everyone should get vaccinated even if they have had Covid-19.
- The system for getting vaccinated is explained here.
- But there are questions as to whether this system is efficient enough because at the current rate Media Hack estimates it will take 18 years to vaccinate 67% of the population.
Every single Johnson & Johnson vaccination destined for the arms of health workers in South Africa is individually prepared by pharmacists and tracked to ensure the cold chain is maintained. The locations where these life-saving vaccines are stockpiled are not disclosed.
At Groote Schuur Hospital, Melanie Maclachlan, a pharmacist from the Desmond Tutu HIV Centre, explains the stringent measures taken to ensure the integrity of the process.
The vaccines are shipped to South Africa in cold storage of between -15°C and - 20°C and stored locally at this low temperature. Once moved from a freezer to a fridge, the temperature rises to between 2°C and 8°C, and the vaccine has to be used within 28 days.
A team of trained experts prepares each syringe by hand. This happens at a secure location. The vaccine vials are pre-packed by the manufacturer in boxes of 20, and each vial can vaccinate two people. The dose is 0.25 ml.
To receive the vaccination, health workers have to register with the Sisonke implementation study. They then receive a voucher and confirmation of the time, date and vaccination site they have to go to.
Maclachlan says that about 30 minutes before vaccination starts they start to fill the syringes. Once the vaccinations are taken out of the fridge, they are viable for two hours.
“Each batch has a temperature logger, and they are monitored every step of the way. So, we know they are being kept stable. We document everything and the data is downloaded from the temperature logger – from the arrival of the shipment to vaccination,” she says.
Even during load shedding, the temperature is controlled. “Every fridge and freezer has back-up. People need to know that it is properly managed,” says Maclachlan.
At the Groote Schuur vaccination site, located on one of the parking levels, there are 18 vaccination cubicles. Sister Rowena Jacobs and sister Celente Malan, are two of the trained vaccinators. Jacobs has been at the hospital for 30 years and she is the operational manager at the Cardio-Thoracic Surgery Unit. Malan is a clinical facilitator, trainer and psychiatric sister.
Jacobs says she nearly died of Covid-19 in June. She was hospitalised for ten days. “It was extremely mentally challenging; so very uncertain. It has taken me eight months to recover and get to the place where I am today.”
Three weeks ago, she was vaccinated. It is recommended that all people are vaccinated, whether they have had Covid-19 or not.
“One of my staff members was a bit hesitant and told me that if he dies, I should look after his child. I said, ‘Make an adult decision and get vaccinated. Make the correct choice and you can take care of your own child’.”
Malan said she wanted to enrol and take the course to vaccinate others, to learn more about the vaccine, and to encourage others to take the vaccine with the knowledge she gained.
Each syringe they receive has a sticker with a code on it. Before a person is vaccinated, they have to enter the details of the person on the Sisonke database and match the code to the person, their identity number and the voucher they received. “No one else can go into the database on our behalf and vaccinate someone else,” she explains.
This is done not only for security reasons, but also so that they can trace the batch number to who received what and when, and to trace any adverse events, if there are any. The system is also paperless.
Sister Rowena Jacobs from Groote Schuur Hospital shows the information sheet and the vaccination card health workers receive after vaccination. Photo: Elsabé Brits
Experts are on site to observe all those who have been vaccinated for 15 minutes, to intervene if there are any serious side effects, such as allergic reaction, which is very rare.
Malan says one might have redness, tenderness and some numbness in the arm. Other side effects might include fatigue, fever, muscle ache or headache. However, one does not necessarily have any side-effects.
“I had no side effects, nothing. It is the best thing you can do for yourself,” says Jacobs.
But can this system scale up?
Government estimates that we need to vaccinate 67% of the population, about 40 million people. At this point, it is estimated (or hoped) that the virus will no longer be able to sustain itself. (But the number is more of a guess than good maths and no one knows Covid’s future trajectory.) At the current rate of vaccination Media Hack estimates it will take 18 years.
The New York Times Covid vaccination tracker says that 381 million vaccine doses have been administered worldwide as of 16 March. South Africa has administered just under 150,000 of these doses, reaching 0.3% of our population. By comparison Rwanda has administered 257,000 doses and reached over 2% of its population. Morocco has used just under 6 million vaccine doses and already reached 12% of its population!
On the Drug Information mailing list that he runs, UKZN pharmacologist Andy Gray wrote: “Although Sisonke plans to allocate 70% of doses to public sector healthcare workers and 30% to private sector, there are persistent complaints about delays in accessing the vouchers needed to obtain vaccination.”
The voucher system is likely only one bottleneck; vaccine supply is another. Supply took a severe hit when government made the controversial decision not to use the one million AstraZeneca vaccine doses delivered to the country earlier this year because of concerns over efficacy. Leading vaccinologists, like Shabir Madhi, have argued that these concerns are misplaced.
UPDATE: Andy Gray wrote in response to this article:
“GroundUp asks the question: But can this system scale up? The answer is that it should not, in its entirety. The next phase, using commercial stock of vaccines approved by the South African Health Products Regulatory Authority (SAHPRA), will not need the level of documentation and control that is needed for a SAHPRA-approved clinical study [as is the current case for the Johnson & Johnson vaccine - Editor]. Care will still be needed to ensure proper transport, storage and administration of the vaccine, but that applies to every vaccination, and in fact to all medicines. Some elements will, nonetheless, be useful. A paperless system is still envisaged, but not necessarily a pre-registration and voucher system. The key will be to offer the public as many options as possible, geographically and time-wise. The systems for reporting adverse events following immunization will also remain in place. It would be useful for SAHPRA to start to report on the number and types of adverse events reported to COVI-Vig [SAHPRA’s Covid monitoring system - Editor], for instance.”
An empty vial of the Johnson & Johnson vaccine which is part of the Sisonke implementation study to vaccinate health care workers in South Africa. One vial is enough to vaccinate two people and one box contains 20 vials. Picture: Elsabé Brits
Support independent journalism
Donate using Payfast

Next: Court slams City of Cape Town for barring access to homeless camp
Previous: Gqeberha roads blocked by protesters demanding jobs
© 2021 GroundUp. This article is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
You may republish this article, so long as you credit the authors and GroundUp, and do not change the text. Please include a link back to the original article.
We put an invisible pixel in the article so that we can count traffic to republishers. All analytics tools are solely on our servers. We do not give our logs to any third party. Logs are deleted after two weeks. We do not use any IP address identifying information except to count regional traffic. We are solely interested in counting hits, not tracking users. If you republish, please do not delete the invisible pixel.